

On January 13, 2024, Professor Wang Xinghuan from the Department of Urology of Zhongnan Hospital of Wuhan University published the latest research "USP43 stabilizes c-Myc to promote glycolysis and metastasis in bladder cancer" on Cell Death&Disease, focusing on the regulation of c-Myc protein in bladder cancer[1]. In this study, a siRNA library targeting ubiquitin enzyme was first used to screen a ubiquitin enzyme, USP43, which can regulate glycolysis and c-Myc transcriptional activity. Subsequent functional experiments and molecular studies revealed that USP43/c-Myc positive feedback loop promoted the metastasis of bladder cancer. (For detailed preliminary research, please refer to: Nature Communications | DNA polymerase POLD1 promotes promotion and metathesis of blade cancer by stabilizing MYC, Oncogene | TRAIP suppresses bladder cancer progression by catalyzing K48-linked polyubiquitination of MYC)

As is well known, a prominent feature of tumor cells is a specific shift towards aerobic glycolytic metabolism, a phenomenon known as the Warburg effect. In bladder cancer, the amplification and activation of the classic proto-oncogene MYC is the key factor leading to this transformation. Targeted treatment of the protein c-Myc encoded by MYC is still a huge challenge. Therefore, more detailed c-Myc regulation mechanism needs to be analyzed to develop selective targeted treatment strategies.
In this study, on the basis of previous screening of deubiquitinating enzymes that regulate tumor cell glycolysis[2], the deubiquitinating enzyme library was further used to screen out the deubiquitinating enzyme USP43 that positively regulates c-Myc transcriptional activity, which is highly expressed in bladder cancer and associated with poor prognosis. Subsequent functional experiments showed that USP43 could promote the glycolysis and metastasis of bladder cancer cells.

In terms of molecular mechanism, USP43 mainly removes the ubiquitin chain modification of lysine at positions 148 and 289 of c-Myc through deubiquitinase activity, thereby stabilizing the c-Myc protein. In addition, the up-regulated USP43 protein in bladder cancer also increased the chance of its interaction with c-Myc, interfering with the binding and degradation of c-Myc by FBXW7. It is worth noting that this study also found that c-Myc can act as a transcription factor of USP43 and activate the transcription of USP43, thus forming a positive feedback loop in bladder cancer. This loop helps to accelerate the glycolysis and metastasis of bladder cancer cells.
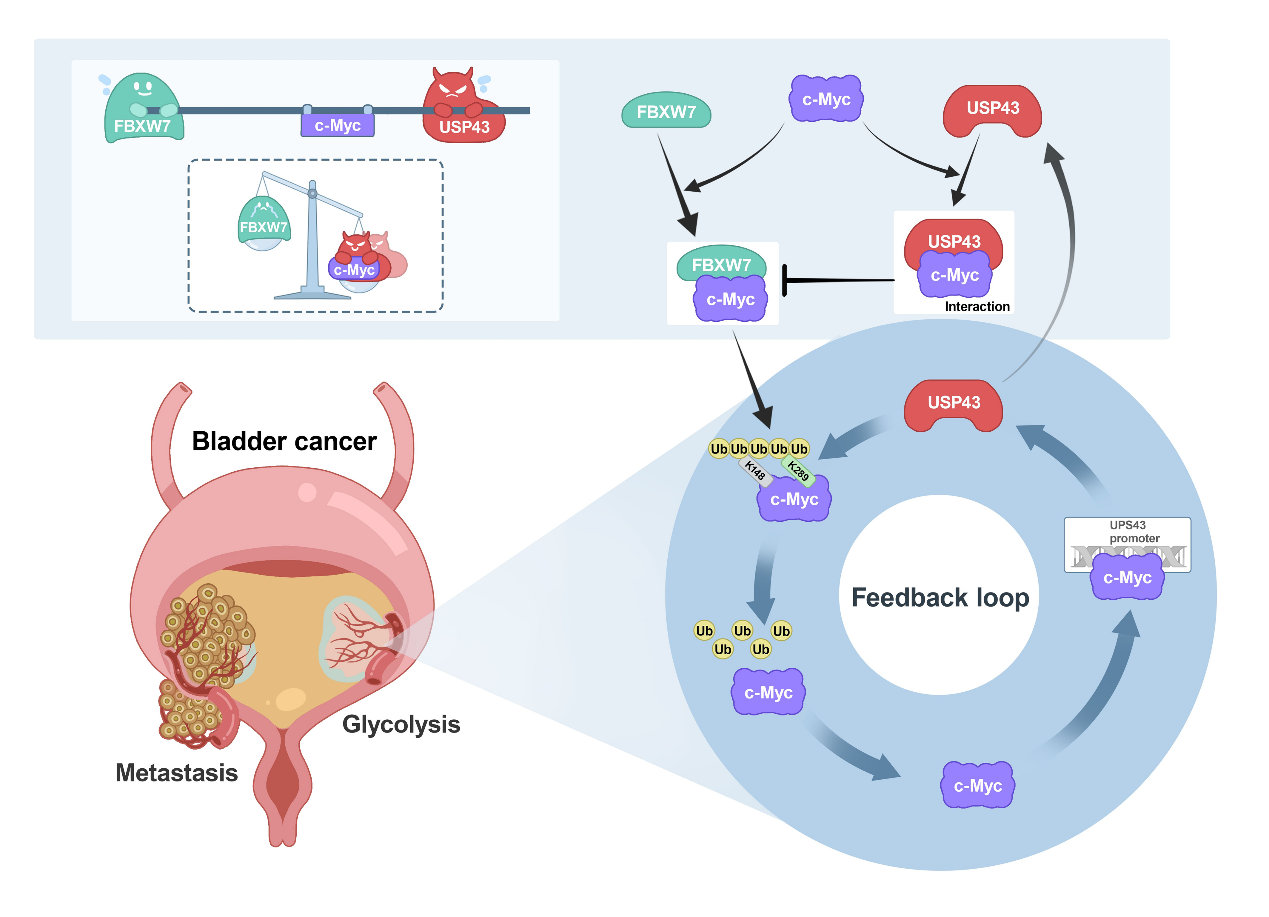
In conclusion, this study has revealed the mechanism of USP43 regulation of glycolysis and c-Myc transcriptional activity, enriched the study of the pathogenesis of bladder cancer, and provided a new potential target for the targeted therapy of bladder cancer. Notably, drug development targeting USP43 requires not only inhibiting its deubiquitase activity, but also degrading the USP43 protein or disrupting its interaction with c-Myc for optimal efficacy. The first author of this paper is Li Mingxing, a doctoral student in the Department of Urology at Zhongnan Hospital of Wuhan University. The project has received research funding support from the National Natural Science Foundation of China, Hubei Provincial Department of Science and Technology, and Zhongnan Hospital of Wuhan University.
References:
[1] Li, M., Yu, J., Ju, L. et al. USP43 stabilizes c-Myc to promote glycolysis and metastasis in bladder cancer. Cell Death Dis 15, 44 (2024). doi:10.1038/s41419-024-06446-7.
[2] Tu R, Kang W, Yang M, et al. USP29 coordinates MYC and HIF1α stabilization to promote tumor metabolism and progression. Oncogene. 2021;40(46):6417-6429. doi:10.1038/s41388-021-02031-w.
Links:
https://www.nature.com/articles/s41419-024-06446-7