

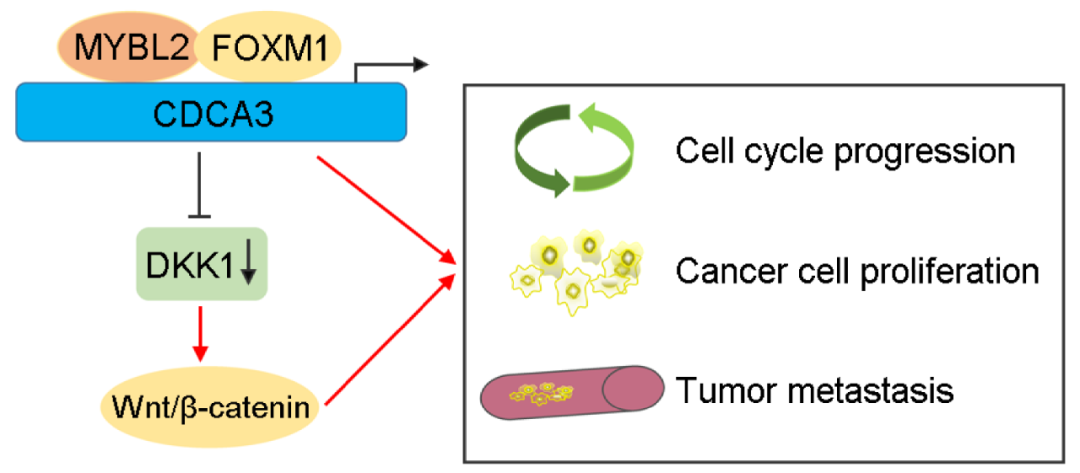
On September 7, 2022, Professor Wang Xinghuan of the Department of Urology, Zhongnan Hospital of Wuhan University, and his team published the latest research results "MYBL2 promotes proliferation and metastasis of bladder cancer through Oncogene, a benchmark journal in the field of oncology. transactivation of CDCA3"[1]. This study shows that MYBL2 can bind to the promoter of CDCA3 and trans activate CDCA3, and can also interact with FOXM1 to jointly regulate the transcription of CDCA3, thereby promoting the proliferation and metastasis of bladder cancer cells. In addition, MYBL2/FOXM1 and CDCA3 may activate Wnt by inhibiting DKK1/ β- Catenin signaling pathway, thus accelerating the proliferation and metastasis of bladder cancer cells.

In previous studies, the research team has found that CDCA3 is an oncogene that can accelerate the proliferation and metastasis of bladder cancer cells. Patients with high levels of CDCA3 expression tend to have poor prognosis[2], but the exact mechanism of CDCA3 affecting the proliferation and metastasis of bladder cancer has not yet been revealed. In this study, the research team further studied the regulatory mechanism of MYBL2, the upstream transcription factor of CDCA3, on CDCA3 and its biological function in bladder cancer.
The research team first confirmed that MYBL2 increased in bladder cancer and its long-term prognosis became worse in the public database and the samples of bladder cancer radical surgery in the Department of Urology of Zhongnan Hospital, and detected the expression changes of MYBL2 in bladder cancer tissues and cells at the transcriptional and protein levels. The results showed that MYBL2 expression was significantly up-regulated in bladder cancer tissues. For patients with high-grade tumors, T2-T4 tumors or progressive diseases, MYBL2 expression was higher in bladder cancer tissues, and its expression was significantly negatively correlated with the tumor specific survival rate of patients.
Phenotypic experiment showed that knockdown MYBL2 could significantly inhibit the proliferation and metastasis of bladder cancer cells, and cause G2 phase arrest of bladder cancer cells; However, overexpression of MYBL2 will promote the proliferation and metastasis of bladder cancer cells. Furthermore, it was confirmed through chromatin immunoprecipitation experiments and dual luciferase reporter gene experiments that MYBL2 can directly bind to the promoter region of CDCA3 and regulate its transcription.
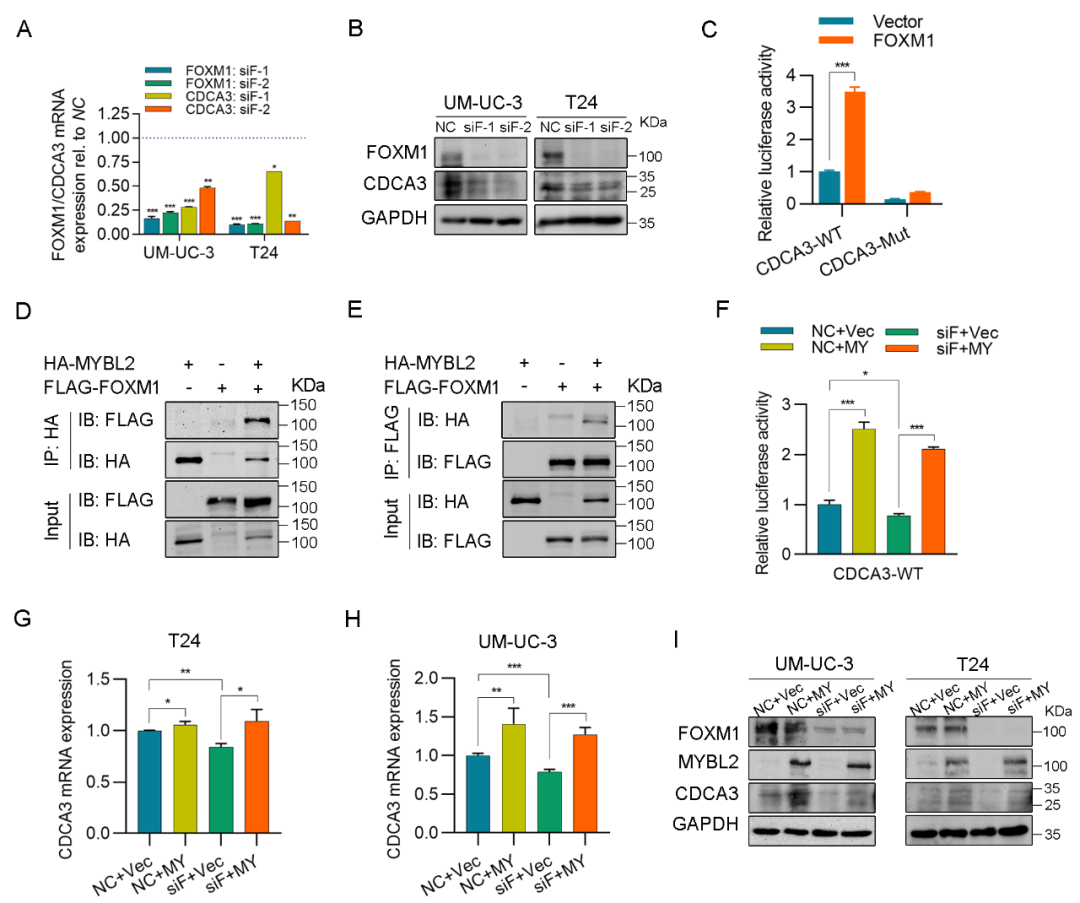
In terms of mechanism, this study found that MYBL2 and CDCA3 regulate Wnt by inhibiting factor DKK1/ β- Catenin signaling pathway, and then affect the proliferation and metastasis of bladder cancer cells. On the other hand, MYBL2 can interact with FOXM1 to jointly regulate the transcription and expression of CDCA3, and then affect the malignant phenotype of bladder cancer cells.
Through systematic research on MYBL2 and CDCA3, this study revealed new carcinogenic targets of bladder cancer, enriched the research on pathogenesis of bladder cancer, and provided new ideas for prognosis judgment and comprehensive treatment of bladder cancer. The first authors of this paper are Liu Wei and Shen Dexin, doctoral students in the Department of Urology at Zhongnan Hospital of Wuhan University. The project has received research funding support from the National Natural Science Foundation of China, Hubei Provincial Department of Science and Technology, and Zhongnan Hospital of Wuhan University.
References:
1. Liu W, et al., MYBL2 promotes proliferation and metastasis of bladder cancer through transactivation of CDCA3. Oncogene. 2022, doi: 10.1038/s41388-022-02456-x. Epub ahead of print.
2. Shen D, et al., The inhibitory effect of silencing CDCA3 on migration and proliferation in bladder urothelial carcinoma. Cancer Cell Int. 2021, 21(1):257. doi: 10.1186/s12935-021-01969-x.
Links:
https://www.nature.com/articles/s41388-022-02456-x